Strategic Value Assessment of Kelun Biotech's Focus on HER2 ADC Drug Resistance Scenarios
Unlock More Features
Login to access AI-powered analysis, deep research reports and more advanced features
About us: Ginlix AI is the AI Investment Copilot powered by real data, bridging advanced AI with professional financial databases to provide verifiable, truth-based answers. Please use the chat box below to ask any financial question.
Related Stocks
Based on the collected information, I now provide you with a systematic and comprehensive analysis report.
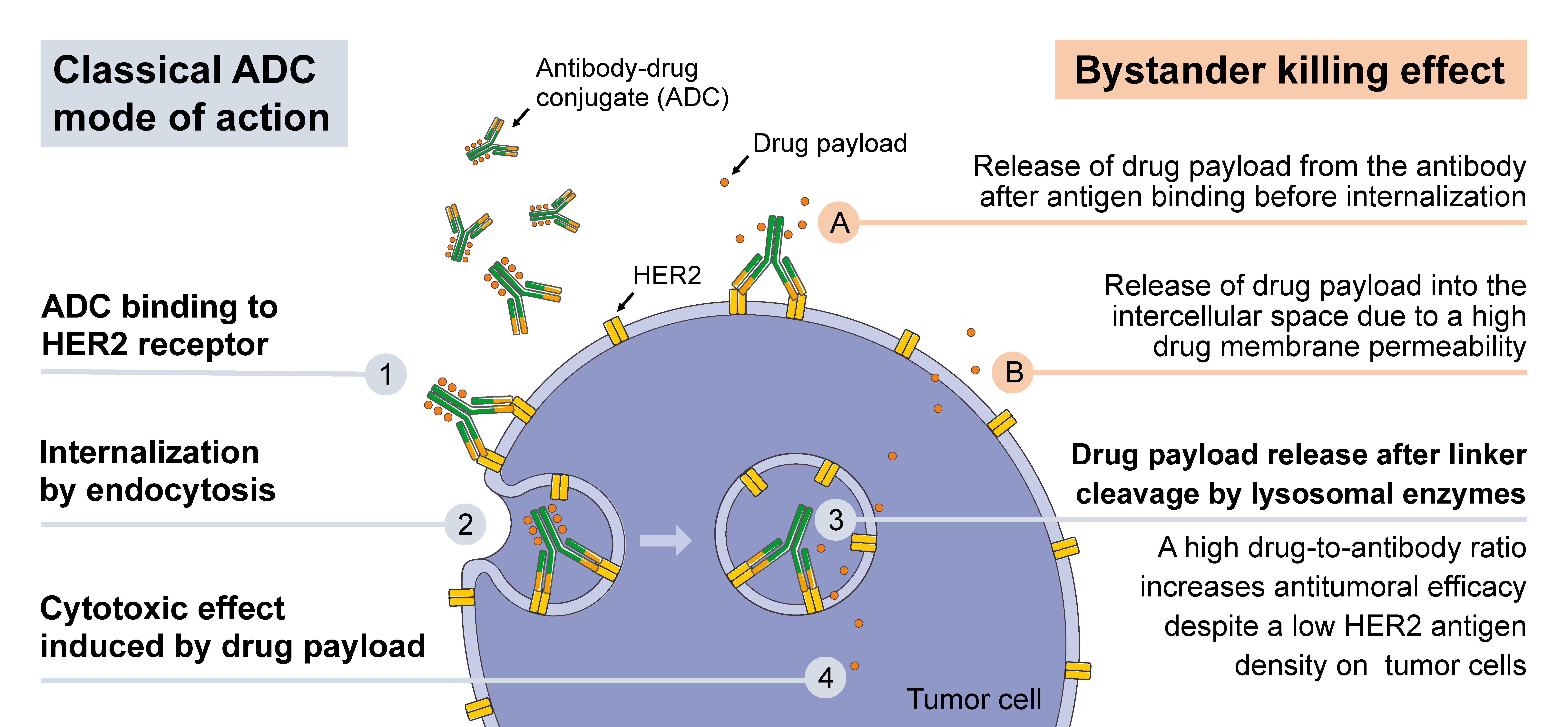
HER2-targeted antibody-drug conjugates (ADCs) have become one of the most commercially valuable and technologically challenging tracks in breast cancer treatment. Since the launch of Enhertu (trastuzumab deruxtecan, T-DXd), this drug has redefined the treatment standard for HER2-positive breast cancer with its breakthrough efficacy, and successfully expanded its indications to the HER2-low expression field, forming a comprehensive replacement trend for traditional HER2-targeted therapies [1][2].
From a global competitive landscape perspective, the current HER2 ADC market exhibits the following characteristics:
| Drug | Developer | Toxin Type | DAR | Approved Indications | Features |
|---|---|---|---|---|---|
Enhertu |
Daiichi Sankyo/AstraZeneca | Dxd (Topoisomerase I inhibitor) | 8 | HER2+ breast cancer, HER2-low expression breast cancer, gastric cancer, lung cancer | Leading efficacy, higher ILD risk |
T-DM1 |
Roche | DM1 (Microtubule inhibitor) | 3.5 | Second-line HER2+ breast cancer | Good safety but limited efficacy |
Disitamab Vedotin |
Rongchang Biotech | MMAE (Microtubule inhibitor) | 4 | Gastric cancer, urothelial carcinoma | Differentiated indication layout |
A166 |
Kelun Biotech | Duo-5 (MMAF derivative) | 2 | Second-line HER2+ breast cancer | Potentially optimal safety |
Enhertu has established significant advantages in clinical efficacy through its unique molecular design (high DAR value of 8, potent topoisomerase I inhibitor Dxd, and prominent bystander effect). In the DESTINY-Breast03 study, Enhertu demonstrated overwhelming efficacy advantages over T-DM1, with a median PFS of 28.8 months, becoming the new standard for second-line treatment of HER2-positive breast cancer [1].
However, Enhertu still faces several challenges in clinical application:
- Safety Risks: The incidence of interstitial lung disease (ILD) is approximately 2.9%-8.8%, and some patients discontinue treatment due to severe toxicity [3][4]
- Drug Resistance Issue: With the popularization of Enhertu in first-line treatment, treatment options after resistance will become an increasingly urgent clinical need
- Administration Restrictions: Has embryonic toxicity, contraindicated in pregnant patients
Kelun Biotech’s Botuzumab (A166, trade name: Shutailai®) was approved for marketing by China’s NMPA on October 17, 2025, as the first domestic ADC drug approved for second-line treatment of HER2-positive breast cancer [5][6].
| Component | A166 Design | Comparison with Enhertu |
|---|---|---|
Antibody |
Trastuzumab | Identical |
Toxin |
Duo-5 (MMAF derivative, microtubule inhibitor) | Enhertu uses Dxd (topoisomerase I inhibitor) |
Linker |
Protease-cleavable linker | Both are cleavable designs |
DAR |
2 | Enhertu is 8 |
Conjugation Method |
Random lysine conjugation | Site-specific cysteine conjugation |
A166 adopts a
| Efficacy Endpoint | A166 | T-DM1 | Magnitude of Advantage |
|---|---|---|---|
| Median PFS | 11.1 months |
4.4 months | 152% increase |
| Objective Response Rate (ORR) | 76.9% |
53.0% | 23.9 percentage points increase |
| Complete Response Rate | Approximately 2.5% | 0% | - |
This study enrolled patients with HER2-positive unresectable or metastatic breast cancer who had previously received trastuzumab and taxane-based therapy. The results showed that A166 achieved improvements in the primary endpoint PFS that were both statistically and clinically significant [5][6].
In heavily pretreated HER2+ breast cancer patients (4.8mg/kg dose group):
- ORR reached 73.9%
- Median PFS was 12.3 months
- Disease Control Rate (DCR) was 100%
Notably, the median number of prior anti-HER2 treatment lines for enrolled patients reached
The most prominent differentiated advantage of A166 lies in its
| Safety Endpoint | A166 | Enhertu | SHR-A1811 |
|---|---|---|---|
| Grade ≥3 Adverse Reactions | 49.4% |
67.5% | 50% |
| Serious Adverse Reactions | 4.9% |
37.5% | - |
| Interstitial Lung Disease (ILD) | 0% |
2.9%-8.8% | - |
| Discontinuation Due to Adverse Reactions | 5.7% |
12.5% | - |
A 0% ILD incidence is A166’s most notable safety advantage, which holds important clinical significance for breast cancer patients requiring long-term treatment [3][4][7].
As Enhertu has become the standard second-line treatment for HER2-positive breast cancer globally, treatment options after resistance are becoming an increasingly urgent unmet clinical need. According to the latest research presented at the 2025 ASCO Annual Meeting, ADC resistance mechanisms mainly include [9]:
- High Expression of Drug Efflux Transporters: High expression of ABCB1 and ABCC1 is significantly associated with shorter treatment duration of T-DXd
- Changes in Target Antigen Expression: Changes in ERBB2 amplification status affect ADC efficacy
- Alterations in Intracellular Drug Metabolic Pathways: Lysosomal dysfunction, internalization impairment
- Median time to next treatment (TTNT) for ERBB2-amplified patients receiving T-DXd reached 22.5 months
- Median TTNT for the non-amplified group was only 6.4 months
- Patients with high ABCB1 expression had a 30% increased risk of death (HR=1.30, p=0.002)
In the face of Enhertu’s strong advancement in first-line therapies, Kelun Biotech has adopted a differentiated strategy of
- Clear Indication Positioning: Position A166 as a “backup option” after resistance to mainstream drugs such as Enhertu
- Launch Specialized Clinical Studies: An open-label, multi-center Phase II clinical study has been initiated for HER2+ unresectable or metastatic breast cancer patients who have received topoisomerase inhibitor ADC therapy
- Multi-Cancer Expansion: In addition to breast cancer, indications for gastric cancer, non-small cell lung cancer, colorectal cancer, etc., are under development
This strategic layout reflects Kelun Biotech’s
- In the first-line market where Enhertu has built strong barriers, the commercial certainty of direct competition is low
- Differentiated resistance backup scenarios have clearer commercial value
- Form a synergistic rather than competitive market pattern with its own and similar products
The fundamental difference between A166 and Enhertu in the selection of cytotoxic drugs provides a theoretical basis for overcoming drug resistance:
| Feature | A166 (MMAF) | Enhertu (Dxd) |
|---|---|---|
Drug Type |
Microtubule inhibitor | Topoisomerase I inhibitor |
Mechanism of Action |
Inhibits microtubule polymerization, blocks mitosis | Induces DNA double-strand breaks |
Cross-Resistance Risk |
Theoretically low | Theoretically low |
Bystander Effect |
Exists | Potent |
According to research, due to different mechanisms of action, the
A166’s zero ILD incidence is particularly important for patients requiring subsequent treatment:
- Enhertu treatment-related ILD risk limits patients’ long-term medication and treatment options
- A166’s safety profile makes it more suitable as a subsequent treatment option
- A lower rate of serious adverse reactions helps maintain patients’ quality of life
The Phase I study included 20.7% of patients who had previously received HER2-ADC therapy, and A166 still demonstrated efficacy in this subgroup, suggesting its application potential in ADC-pretreated patients [7][8].
- With the popularization of Enhertu globally, the post-resistance treatment market will continue to expand
- The base of HER2-positive breast cancer patients is stable, with approximately 200,000 new cases each year
- HER2-positive breast cancer patients in China account for approximately 20%-25% of all breast cancer patients
| Enterprise | Product | Strategic Positioning | Differentiated Advantage |
|---|---|---|---|
| Kelun Biotech | A166 | Focus on drug resistance scenarios | Zero ILD safety |
| Baili Tianheng | T-Bren | Directly compete with Enhertu | Cross-cancer layout |
| Rongchang Biotech | Disitamab Vedotin | Differentiated indications | Gastric cancer, urothelial carcinoma |
| Hengrui Medicine | SHR-A1811 | Multi-indication expansion | Combination regimen development |
Kelun Biotech has reached a strategic cooperation with Merck, licensing SKB264 (global rights excluding Greater China), SKB315 (global rights), and seven preclinical ADC assets to Merck, with a total transaction value of up to
Kelun Biotech’s ADC pipeline layout exhibits good synergy:
- SKB264(TROP2 ADC): Targets indications such as TNBC, NSCLC, etc.
- A166(HER2 ADC): Focuses on breast cancer drug resistance scenarios
- SKB315(CLDN18.2 ADC): Targets solid tumors such as gastric cancer
A multi-target layout can form synergies in treatment strategies, providing sequential treatment options for patients.
- Phase II Clinical Results in Drug Resistance Scenarios: Still need verification from Phase III studies with larger sample sizes
- Long-Term Safety Data: Need longer-term follow-up data
- Biomarker Screening: How to accurately identify the patient population that can benefit most from A166
- Enhertu Indication Advancement: If Enhertu achieves breakthroughs in neoadjuvant/adjuvant therapy, the characteristics of drug-resistant populations may change
- Competition from Other ADCs: Competitive pressure from products such as Hengrui’s SHR-A1811 and Baili Tianheng’s T-Bren
- Technology Iteration Risk: Potential impact from new ADC technologies (such as bispecific antibody ADCs)
- Medical Insurance Negotiation Pressure: Balancing innovative drug pricing and medical insurance access
- Production Capacity Supply: Complexity of ADC drug production
- Academic Promotion: Clinical concept education for drug resistance scenarios
Based on the above analysis, Kelun Biotech’s focus on HER2 ADC drug resistance scenarios has the following strategic value:
| Dimension | Assessment | Rating |
|---|---|---|
Scientific Basis |
Mechanistic differences between MMAF and Dxd, theoretically avoid cross-resistance | ★★★★☆ |
Clinical Data |
Excellent Phase III head-to-head data, significant safety advantages | ★★★★★ |
Strategic Positioning |
Clear differentiated competition strategy, avoids direct conflict with Enhertu | ★★★★★ |
Market Space |
Real and continuously growing demand for drug resistance treatment | ★★★★☆ |
Execution Capability |
Merck cooperation validates platform value, leading R&D progress | ★★★★★ |
- Complete Registration Clinical Studies in Drug Resistance Scenarios: Need to initiate and complete Phase III clinical studies for ADC-pretreated patients
- Establish an Accurate Patient Stratification System: Screen the most beneficial population based on biomarkers (such as ABCB1 expression, ERBB2 amplification status)
- Form a Clear Clinical Positioning: Establish the status of “A166 after Enhertu progression” in treatment guidelines
- Continuous Academic Promotion: Establish clinical awareness through clinical data release and academic cooperation
Kelun Biotech’s “focus on drug resistance scenarios” strategy reflects a profound understanding of the competitive landscape of China’s innovative drugs. In the first-line market where Enhertu has established strong barriers, this
It is recommended to pay attention to the following catalysts:
- Release of Phase II/III clinical data in drug resistance scenarios
- Results of medical insurance negotiations
- Progress of international clinical studies
- Follow-up advancement of cooperation with Merck
[1] China Pharmaceutical Innovation and Research Development Association - HER2 ADC Track: Survival of Domestic Players? (https://www.phirda.com/artilce_41347.html)
[2] Tencent News - HER2 ADC Track: Survival of Domestic Players? (https://news.qq.com/rain/a/20260112A01DTT00)
[3] SPD Bank International - Kelun Biotech (6990.HK): ADC Leader on the Verge of Commercialization, Promising Future (https://www.spdbi.com/)
[4] Industrial Securities - ADC Pioneer Goes Global, Innovative Platform Has Great Potential (https://pdf.dfcfw.com/pdf/H3_AP202312041613222468_1.pdf)
[5] Smart Pharma Circle - Kelun Biotech’s Innovative Drug Botuzumab Approved (https://synapse.zhihuiya.com/blog/)
[6] Cancer-Free Home - Another Domestic HER2 ADC New Drug Approved (https://m.tumormed.com/aizhengzhiliao/7814.html)
[7] Kelun Biotech Official Website - News Center (http://www.kelun-biotech.com/newsCenter.aspx)
[8] NPJ Breast Cancer - Phase I study of A166 (https://www.nature.com/)
[9] 2025 ASCO Annual Meeting - ADC Continues to Lead Cancer Treatment (https://zh.tri-apex.com/info/insight/720.html)
Report Compiled by: Jinling AI Financial Analysis Team
Data Cut-Off Date: January 14, 2026
Insights are generated using AI models and historical data for informational purposes only. They do not constitute investment advice or recommendations. Past performance is not indicative of future results.
About us: Ginlix AI is the AI Investment Copilot powered by real data, bridging advanced AI with professional financial databases to provide verifiable, truth-based answers. Please use the chat box below to ask any financial question.
