Altimmune (ALT) Post-$5 Valuation: Catalysts, Clinical Data, and Risk Analysis
Unlock More Features
Login to access AI-powered analysis, deep research reports and more advanced features
About us: Ginlix AI is the AI Investment Copilot powered by real data, bridging advanced AI with professional financial databases to provide verifiable, truth-based answers. Please use the chat box below to ask any financial question.
Related Stocks
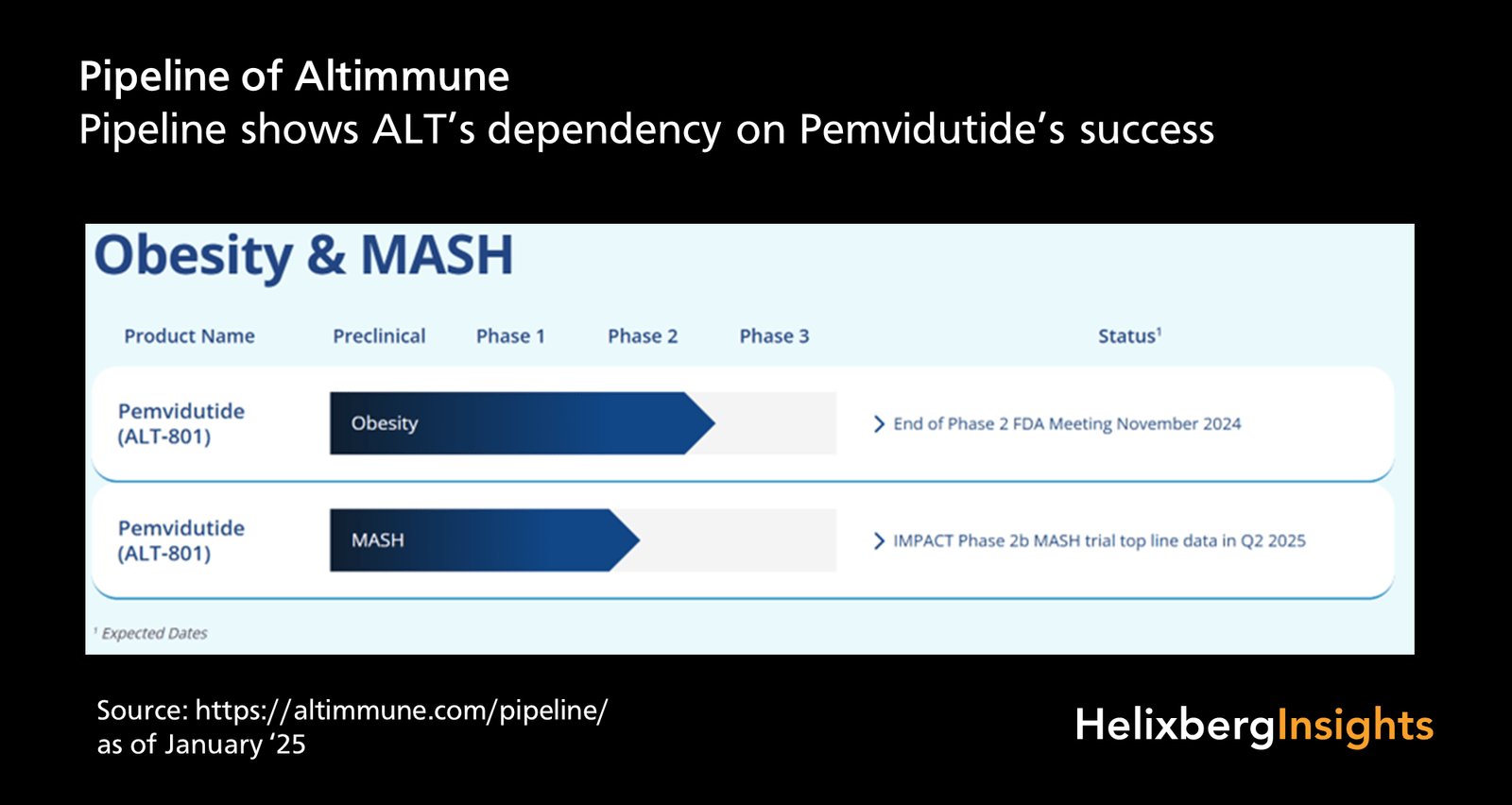
Altimmune’s (ALT) stock price recently exceeded $5, validating the core claim from a November 23 Reddit post [6]. The company’s lead drug pemvidutide showed 58%/52% MASH resolution in 24-week trials [4], though fibrosis improvement was not statistically significant—consistent with delayed antifibrotic effects of GLP-1 therapies [4]. Upcoming Q4 2025 catalysts (48-week trial data, FDA meeting) are expected to drive further valuation changes [5]. Analysts forecast 250% upside [3], supported by BioSpace’s estimate of $1B+ peak revenue for MASH [2].
- The 24-week trial’s fibrosis result may not be definitive, as antifibrotic effects often take longer [4].
- The Reddit post’s 21% short interest claim remains unconfirmed [3].
- Analyst upside depends on successful catalyst outcomes (trial data, FDA approval) [3].
- Positive 48-week trial data could unlock significant valuation upside [3].
- Strategic partnerships or licensing deals may boost investor confidence [4].
Risks: - Clinical trial failure (e.g., no fibrosis improvement in 48-week data) [4].
- FDA rejection or delayed approval [5].
- Valuation pullback if catalysts do not meet expectations [3].
- Current price: $5.26 (as of 2025-11-29) [0].
- Clinical: 58% MASH resolution (1.2mg dose) in Phase IIb [4].
- Catalysts: Q4 2025 48-week IMPACT trial data and FDA End of Phase 2 meeting [5].
- Analyst outlook: 250% upside predicted [3].
Insights are generated using AI models and historical data for informational purposes only. They do not constitute investment advice or recommendations. Past performance is not indicative of future results.
About us: Ginlix AI is the AI Investment Copilot powered by real data, bridging advanced AI with professional financial databases to provide verifiable, truth-based answers. Please use the chat box below to ask any financial question.