In Vivo CAR-T Technology Route Investment Value Analysis
Unlock More Features
Login to access AI-powered analysis, deep research reports and more advanced features
About us: Ginlix AI is the AI Investment Copilot powered by real data, bridging advanced AI with professional financial databases to provide verifiable, truth-based answers. Please use the chat box below to ask any financial question.
Related Stocks
Based on my in-depth research on the In Vivo CAR-T technology route, I will provide you with a comprehensive investment value analysis.
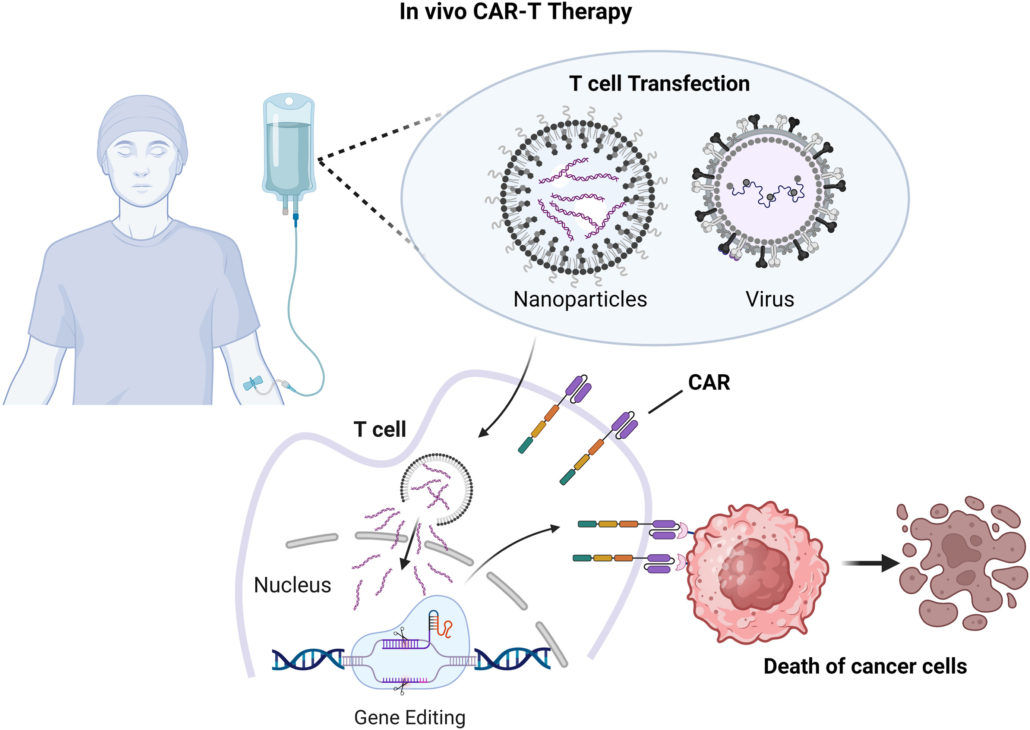
In Vivo CAR-T technology represents a major breakthrough in the field of cell therapy. By directly generating CAR-T cells in the patient’s body, it completely changes the complex process of traditional CAR-T therapy that requires ex vivo cell manufacturing. This technical route has revolutionary advantages:
- Significantly Reduced Manufacturing Costs: Eliminates complex ex vivo operations such as cell separation, modification, and expansion
- Improved Treatment Accessibility: Shift from “personalized customization” to “off-the-shelf products”
- Shorter Treatment Time: From the 2-4 week preparation cycle of traditional CAR-T to a few days
- Rapid Transfection: The LNP platform enables rapid T-cell targeting and transfection [1]
- Repeatable Administration: LNP formulations have better safety and repeatable administration characteristics [2]
- Targeting Precision: T-LNP (T-cell targeting lipid nanoparticle) technology achieves highly selective T-cell targeting
Vivacta Bio’s GT801 released encouraging first-in-human trial data at the 2025 ASH Annual Meeting [1]:
- Uses T-LNP to deliver mRNA encoding anti-CD19 CAR
- Both patients showed high CAR expression in circulating T cells
- Well-tolerated with no obvious off-target effects
- Supports the feasibility of repeat administration
CREATE Medicines’ MT-303 also initiated clinical trials in first-line treatment of hepatocellular carcinoma [3], demonstrating the application potential of LNP technology in solid tumors.
- Stable Integration: Lentiviral vectors enable stable integration of genes, providing long-lasting therapeutic effects
- High Transduction Efficiency: Significant advantage in transducing T cells in vivo
Kelonia Therapeutics’ KLN-1010 achieved breakthrough progress in BCMA multiple myeloma treatment [4]:
- 100% MRD-negative response rate (4 patients)
- Longest follow-up of 5 months, all patients maintained response
- No grade 3 or higher cytokine release syndrome (CRS) or immune effector cell-associated neurotoxicity syndrome (ICANS)
- Excellent safety profile
-
BMS Acquires Orbital Therapeutics - $1.5 Billion[5]
- Acquisition Core: OTX-201 (next-generation in vivo CAR-T therapy)
- Strategic Significance: Strengthens CAR-T layout in the field of autoimmune diseases
-
Gilead Acquires Interius BioTherapeutics - $350 Million[6]
- Acquisition Core: Lentivirus-mediated in vivo CAR-T platform
- Strategic Significance: Deepens cell therapy layout through the Kite division
-
Other M&A Activities with Undisclosed Amounts
- Technical Complementation Needs: Large pharmaceutical companies need to quickly acquire emerging technology platforms through acquisitions
- Market Access Barriers: High threshold for in vivo CAR-T technology, high risk of independent R&D
- Urgency of Patent Layout: Early patent layout is crucial for future market position
- Cost-Effectiveness Considerations: Acquiring mature platforms is more cost-effective than R&D from scratch
-
Clinical Data Catalysts
- Pay attention to key clinical trial data readout time points of various companies
- Data releases at important academic conferences such as ASH and ASCO
- Regulatory milestone events
-
Technology Platform Companies
- LNP delivery technology platform companies (e.g., Acuitas Therapeutics)
- Targeting ligand technology providers
- CDMO service providers
-
Commercial Breakthroughs
- First in vivo CAR-T product approved for marketing
- Indication expansion (from hematological tumors to solid tumors)
- Cost reduction due to mature manufacturing processes
-
Platform Licensing Opportunities
- Licensing cooperation of technology platforms to large pharmaceutical companies
- Commercial cooperation in regional markets
-
Technology Integration and Innovation
- Combination of in vivo CAR-T and gene editing technology
- Application of artificial intelligence in drug design
- Development of personalized medicine
-
Market Pattern Reshaping
- Traditional CAR-T companies transitioning to in vivo technology
- IPO opportunities for emerging technology companies
- Accelerated industry integration
-
Technical Risks
- In vivo delivery efficiency and specificity still need optimization
- Long-term safety and immunogenicity issues
- Solid tumor treatment faces greater challenges
-
Regulatory Risks
- Regulatory path for new technology routes is not yet clear
- Potential impact of safety events
-
Market Risks
- Pricing and medical insurance access challenges
- Continuous improvement of traditional CAR-T technology forms competition
-
Key Investment Directions:
- Companies with differentiated technology platforms
- Projects in clinical data validation phase with good safety
- Mid-to-late stage projects with potential for cooperation with large pharmaceutical companies
-
Timing of Investment:
- Pay attention to clinical data catalyst events
- Use market fluctuations to invest at low points
- Attach importance to the scalability of technology platforms
-
Risk Control Strategies:
- Diversify investment in technical routes
- Pay attention to regulatory progress and safety data
- Set clear exit mechanisms
[1] BioSpace - “Vivacta Bio Announces Promising First-in-Human Results for GT801, an In Vivo CAR-T Therapy, in Non-Hodgkin’s Lymphoma at the 2025 ASH Annual Meeting” (https://www.biospace.com/press-releases/vivacta-bio-announces-promising-first-in-human-results-for-gt801-an-in-vivo-car-t-therapy-in-non-hodgkins-lymphoma-at-the-2025-ash-annual-meeting)
[2] BioSpace - “Acuitas Therapeutics Showcases Collaboration with Children’s Hospital of Philadelphia for Personalized CRISPR Therapy and New LNP Research at ASGCT 2025” (https://www.biospace.com/press-releases/acuitas-therapeutics-showcases-collaboration-with-childrens-hospital-of-philadelphia-for-personalized-crispr-therapy-and-new-lnp-research-at-asgct-2025)
[3] BioSpace - “CREATE Medicines Doses First Patient in Frontline HCC Trial Evaluating MT-303, an In Vivo CAR Therapy” (https://www.biospace.com/press-releases/create-medicines-doses-first-patient-in-frontline-hcc-trial-evaluating-mt-303-an-in-vivo-car-therapy-in-combination-with-standard-of-care-immunotherapy)
[4] BioSpace - “Kelonia Therapeutics Presents First-in-Human Data From Phase 1 inMMyCAR Study of KLN-1010 in vivo BCMA CAR-T Therapy at the 2025 ASH Annual Meeting” (https://www.biospace.com/press-releases/kelonia-therapeutics-presents-first-in-human-data-from-phase-1-inmmycar-study-of-kln-1010-in-vivo-bcma-car-t-therapy-at-the-american-society-of-hematology-ash-2025-annual-meeting)
[5] Yahoo Finance - “Bristol Myers buys Orbital Therapeutics for $1.5 billion” (https://finance.yahoo.com/news/bristol-myers-buys-orbital-therapeutics-111651114.html)
[6] Yahoo Finance - “Gilead’s Kite scoops in vivo CAR-T specialist Interius for $350m” (https://finance.yahoo.com/news/gilead-kite-scoops-vivo-car-105336605.html)
曹操出行管理层自愿限售股份对投资者的意义
Insights are generated using AI models and historical data for informational purposes only. They do not constitute investment advice or recommendations. Past performance is not indicative of future results.
About us: Ginlix AI is the AI Investment Copilot powered by real data, bridging advanced AI with professional financial databases to provide verifiable, truth-based answers. Please use the chat box below to ask any financial question.