Nanoparticle Separation Technology: Market Disruption Analysis and Investment Opportunities
Unlock More Features
Login to access AI-powered analysis, deep research reports and more advanced features
About us: Ginlix AI is the AI Investment Copilot powered by real data, bridging advanced AI with professional financial databases to provide verifiable, truth-based answers. Please use the chat box below to ask any financial question.
Related Stocks
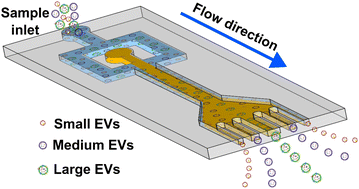
Researchers at the University of Oulu in Finland have developed a breakthrough
The Finnish research team, led by S. Abdorahimzadeh as part of doctoral research examining electroviscoelastic and electroinertial methods, developed a microfluidic approach that integrates two physical phenomena:
| Component | Mechanism | Advantage |
|---|---|---|
Electrophoretic Slip |
Electric fields generate lift forces on particles in motion | Enables precise particle manipulation without complex nanofluidic channels |
Viscoelastic Lateral Forces |
Arise specifically in non-Newtonian fluids | Creates separation forces unavailable in conventional aqueous solutions |
- Particle Purity Improvement: 30-50% improvement for polystyrene beads; >20% for cancer-cell extracellular vesicles (EVs) [1]
- Scalability: Operates at lower pressures in standard microchannels, avoiding clogging issues
- Processing Speed: Significantly faster than traditional ultracentrifugation and chromatography methods
- Sample Integrity: Gentle processing preserves EV integrity for downstream biomarker analysis
Current nanoparticle and extracellular vesicle isolation methods suffer from significant limitations that create disruption opportunities:
| Current Method | Limitations | Market Pain Point |
|---|---|---|
| Ultracentrifugation | Time-consuming (4-6 hours), low yield, sample damage | $450+ per sample processing cost |
| Size-Exclusion Chromatography | Low throughput, column fouling | Limited scalability |
| Immunoaffinity Capture | High cost, antibody variability | $800-1,500 per test |
| Microfiltration | Poor selectivity, membrane clogging | 60-70% sample loss |
The technology targets multiple high-growth markets:
┌─────────────────────────────────────────────────────────────────────┐
│ ADDRESSABLE MARKET (2025-2035) │
├─────────────────────────────────────────────────────────────────────┤
│ • Liquid Biopsy Market: $7.08B (2025) → $27.42B (2035) │
│ • Circulating Tumor Cells: $13.37B (2025) → $31.14B (2033) │
│ • Nanomedicine Market: $235B (2025) → $724B (2035) │
│ • Microfluidic Devices: $8.14B (2025) → $17.09B (2033) │
│ • EV-Based Liquid Biopsy: $122.7M (2025) → $149.7M (2026) │
└─────────────────────────────────────────────────────────────────────┘
Source: Industry analysis reports [2][3][4][5]
- Higher-purity EV extractionfrom plasma improves sensitivity of liquid biopsy assays
- Reduced sample preparation timefrom hours to minutes
- Lower cost per sampleenables broader clinical adoption
- Early cancer detection represents the fastest-growing liquid biopsy segment
- North America dominates with 46% revenue share in circulating tumor cell (CTC) research [6]
- Regulatory approvals increasing: FDA has cleared multiple liquid biopsy tests since 2024
- Improved isolation of cancer-cell-derived EVsenables better biomarker detection
- Potential for multi-analyte panelscombining ctDNA, EVs, and CTCs
- Enables treatment monitoringwith higher precision
- Quality control for drug-loaded nanoparticlesand liposomes
- Targeted deliveryformulation development
- Pharmacokineticsstudies with improved particle tracking
| Company | Ticker | Relevance | Investment Thesis |
|---|---|---|---|
Danaher Corporation |
NYSE: DHR | Life sciences diagnostics, precision medicine partnerships | Partnership with AstraZeneca for AI-powered diagnostics; broad IVD exposure [9] |
Thermo Fisher Scientific |
NYSE: TMO | Lab equipment, microfluidics, clinical diagnostics | Leading supplier of liquid biopsy and EV isolation products [10] |
Guardant Health |
NASDAQ: GH | Liquid biopsy leader, 38.5% YoY revenue growth Q3 2025 | Expanding early detection portfolio (Galleri test) [11] |
Natera |
NASDAQ: NTRA | cfDNA diagnostics, signatera residual disease testing | Technology-agnostic target for liquid biopsy adoption |
Roche |
SW: ROG | Oncology diagnostics, CTC platforms (CellSearch) | Market leader in clinical diagnostics |
QIAGEN |
NYSE: QGEN | Sample preparation, liquid biopsy consumables | Strong EV and cfDNA isolation portfolio |
CelLBxHealth (ANGLE plc) |
LON: CLBX | CTC isolation technology | Pure-play CTC intelligence company [12] |
| Company | Focus | Funding Status | Investment Consideration |
|---|---|---|---|
Hermes Biosciences |
Automated EV isolation benchtop system | Seed funding (Nov 2025) | Commercial instrument expected 2026; strong Stanford nanofiltration IP [13] |
Menarini Silicon Biosystems |
CTC enrichment (DEPArray) | Private | Acquisition target for larger diagnostics players |
Multiple microfluidic startups |
Lab-on-a-chip POC diagnostics | Various | High-risk, high-reward opportunities |
The microfluidic chip solution market grew from $2.56 billion (2025) to $2.81 billion (2026), with projected growth at 9.75% CAGR reaching $17.09 billion by 2033 [14][15]. Key players include:
- Illumina(sequencing infrastructure)
- 10x Genomics(single-cell analysis)
- Standard BioTools(lab-on-a-chip instruments)
HIGH RETURN POTENTIAL
│
┌────────────────────┼────────────────────┐
│ │ │
│ MICROIUIDIC │ EV ISOLATION │
│ STARTUPS │ FIRMS │
│ • High Growth │ • Strong IP │
│ • Technology risk │ • Clinical need │
│ • $500M+ target │ • $150M+ target │
LOW │ │ │ HIGH
RISK ├────────────────────┼────────────────────┤ RISK
│ │ │
│ LARGE IVD │ NANOMEDICINE │
│ COMPANIES │ COMPOUNDS │
│ • Lower growth │ • Long timelines │
│ • Lower risk │ • Regulatory risk │
│ • $5B+ target │ • $2B+ target │
│ │ │
└────────────────────┼────────────────────┘
│
LOW RETURN POTENTIAL
| Investment Tier | Examples | Allocation | Rationale |
|---|---|---|---|
Core Holdings (60%) |
Danaher, Thermo Fisher, Roche | Diversified exposure across diagnostics value chain; lower volatility; dividend yields | |
Growth Segment (25%) |
Guardant Health, Natera, CelLBxHealth | Direct liquid biopsy/CTC market exposure; higher growth potential | |
Opportunistic (10%) |
Hermes Biosciences (when public), microfluidic IPOs | Early-stage technology exposure; higher risk/reward | |
Hedging (5%) |
Short medical devices ETFs (XHE) | Potential hedge against sector-specific risks |
| Period | Event | Investment Impact |
|---|---|---|
2025-2026 |
Proof-of-concept publications; initial commercial partnerships | Early movers can establish IP positions |
2026-2027 |
Clinical validation studies; regulatory engagement | De-risking for clinical applications |
2027-2029 |
Product commercialization by major IVD players | Revenue growth visibility |
2029-2032 |
Market adoption in clinical settings | Mainstream validation |
2032+ |
Standard-of-care integration | Sustained market leadership |
- FDA clearancesfor EV-based diagnostic tests
- Clinical trial resultsvalidating EV biomarkers for cancer detection
- Major IVD company acquisitionsof EV isolation technology firms
- Reimbursement decisionsby CMS and private insurers for liquid biopsy
- International adoptionin European and Asian markets
- Clinical validation: Requires multi-center studies demonstrating improved patient outcomes
- Regulatory pathway: Novel technology may face extended FDA review periods
- Manufacturing scale-up: Transition from lab to commercial production
- Reimbursement limitations: Insurance coverage gaps may slow adoption
- Competitive disruption: Alternative technologies (e.g., new chromatography methods) could emerge
- Market consolidation: Large IVD players may acquire key intellectual property
- Valuation concerns: High growth expectations may lead to overvaluation
- Funding dependency: Private companies may require multiple financing rounds
- Currency exposure: International revenue subject to FX fluctuations
The Finnish nanoparticle separation technology represents a significant innovation with the potential to disrupt multiple high-growth biomedical diagnostic markets. The
-
Primary Opportunity: Direct exposure to liquid biopsy market growth through established players (Guardant Health, Natera) and CTC technology leaders (CelLBxHealth)
-
Infrastructure Play: Large-cap IVD companies (Danaher, Thermo Fisher) offer lower-risk exposure with potential for technology licensing or acquisition
-
Emerging Opportunities: Monitor private company funding rounds (Hermes Biosciences) and potential IPO pipeline in EV isolation and microfluidic spaces
-
Timeline: Expect commercialization announcements 2026-2027, with significant market penetration 2029-2032
[1] News-Medical - “New nanoparticle separation method boosts biotech and cancer research” (https://www.news-medical.net/news/20260209/New-nanoparticle-separation-method-boosts-biotech-and-cancer-research.aspx)
[2] Towards Healthcare - “Liquid Biopsy Market Size” (https://www.towardshealthcare.com/insightimg/liquid-biopsy-market-size.webp)
[3] GlobeNewswire - “Circulating Tumor Cells Market to Surpass US$ 19.0 Billion” (https://www.globenewswire.com/news-release/2026/02/09/3234675/0/en/Circulating-Tumor-Cells-Market-to-Surpass-US-19-0-Billion-by-2032-as-Liquid-Biopsy-Adoption-Expands-Says-Astute-Analytica.html)
[4] InsightAce Analytic - “Nanomedicine Market Research Report” (https://www.insightaceanalytic.com/report/nanomedicine-market-/1889)
[5] Yahoo Finance - “Microfluidic Devices Market Size to Reach USD 17.09 Billion” (https://finance.yahoo.com/news/microfluidic-devices-market-size-reach-120000961.html)
[6] Research and Markets - “Non-Invasive Liquid Biopsy Market” (https://www.researchandmarkets.com/reports/5925108/non-invasive-liquid-biopsy-market-global)
[7] Fortune Business Insights - “Liquid Biopsy Market Size, Share” (https://www.fortunebusinessinsights.com/liquid-biopsy-market-102506)
[8] Spherical Insights - “Top 20 Global Companies in Nanomedicine Market” (https://www.sphericalinsights.com/blogs/top-20-global-companies-in-nanomedicine-market-2025-2035-competitive-analysis-and-forecast)
[9] Danaher Investor Relations - “Diagnostic Development and Commercialization Partnership” (https://investors.danaher.com/2025-05-29-Danaher-Announces-Diagnostic-Development-and-Commercialization-Partnerhip-to-Scale-Precision-Medicine)
[10] Business Wire - “Thermo Fisher Scientific Showcases Diagnostics Solutions” (https://www.businesswire.com/news/home/20250728341807/en/Thermo-Fisher-Scientific-Showcases-Diagnostics-Solutions-Designed-to-Meet-Evolving-Global-Healthcare-Demands-at-ADLM-2025)
[11] CSIMarket - “Guardant Health Inc Comparisons” (https://csimarket.com/stocks/compet_glance.php?code=GH)
[12] Yahoo Finance - “CelLBxHealth PLC Announces Preliminary Fourth Quarter” (https://finance.yahoo.com/news/cellbxhealth-plc-announces-preliminary-fourth-072600428.html)
[13] Business Wire - “Hermes Biosciences Raises Seed Funding” (https://www.businesswire.com/news/home/20251118339710/en/Hermes-Biosciences-Raises-Seed-Funding-to-Make-Extracellular-Vesicles-a-Practical-Tool-for-Precision-Medicine)
[14] Research and Markets - “Microfluidic Chip Solution Market” (https://www.researchandmarkets.com/reports/6122417/microfluidic-chip-solution-market-global)
[15] EIN Presswire - “Medical Microfluidic Devices Market” (https://www.einpresswire.com/article/889184588/medical-microfluidic-devices-market-expected-to-reach-12-79-billion-by-2030-analysis-by-the-business-research-company)
Insights are generated using AI models and historical data for informational purposes only. They do not constitute investment advice or recommendations. Past performance is not indicative of future results.
About us: Ginlix AI is the AI Investment Copilot powered by real data, bridging advanced AI with professional financial databases to provide verifiable, truth-based answers. Please use the chat box below to ask any financial question.